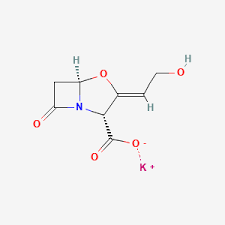
PHARMASTARS completed the registration process of the GABAPENTIN API (Active Pharmaceutical Ingredient) on EDA’s (Egyptian Drug Authority) API white list.
The GABAPENTIN API produced by ZHEJIANG CHIRAL MEDICINE (CHINA) and used by PHARMASTARS for the production of the finished formulation “GABROXIA” is registered in EDQM (European Directorate for the Quality of Medicines and Healthcare) and in USFDA (United State Food and Drug Administration).
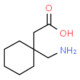
GABAPENTIN is a gamma-amino acid that is cyclohexane substituted at position 1 by aminomethyl and carboxymethyl groups. Used for treatment of neuropathic pain and restless legs syndrome. It has a role as an anticonvulsant, a calcium channel blocker, an environmental contaminant and a xenobiotic.
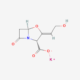
PHARMASTARS completed the registration process of the Diluted Potassium Clavulante API (Active Pharmaceutical Ingredient) on EDA’s (Egyptian Drug Authority) API white list.
The API produced by SHANDONG NEW TIME PHARMACEUTICALS (CHINA) and used by PHARMASTARS for the production of the finished formulations “MEDICLAVE” is registered in EDQM (European Directorate for the Quality of Medicines and Healthcare) and in USFDA (United State Food and Drug Administration)